Molecular Pharmacology
Director: Aiming Yu, Ph.D.
Technical Director: Anthony Martinez
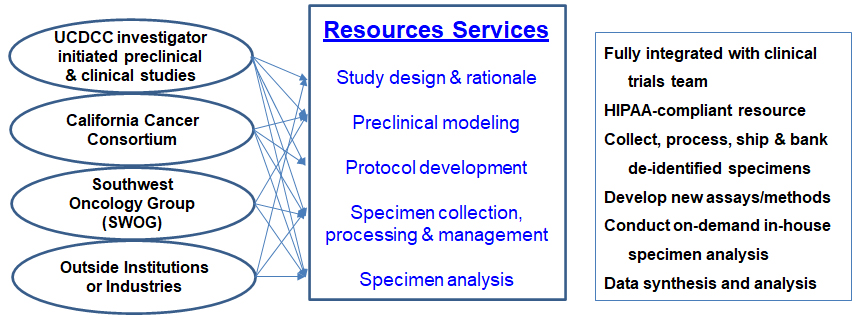
The Molecular Pharmacology Shared Resource, or MPSR, provides a mechanism for high-quality collection, processing and analysis of clinical specimens from clinical trial patients for pharmacokinetic and pharmacodynamics studies. Additionally, the MPSR conducts preclinical modeling studies on novel anticancer agents or therapeutic combinations and implements molecular pharmacological research with clinical impact.
Objectives
Users are generally principal investigators of clinical trials seeking to incorporate translational and/or pharmacokinetic and pharmacodynamic studies into their trial design or generate exploratory or validational preclinical modeling for new agent development or concept proof-of-principle, as well as preclinical investigators to define drug metabolism, pharmacokinetics, and pharmacodynamics properties of new agents. The long-term objectives of this resource are to delineate pharmacological actions of new therapeutics and to identify valid factors that are predictive of therapeutic outcomes.
Specimen management
A dedicated staff research associate conducts clinical specimen collection, processing and management for most UC Davis Comprehensive Cancer Center clinical trials, which often require unique preparation techniques specific to individual clinical trial protocols. The shared resource administers and maintains a secure, independent specimen database. All processed specimens are logged for tracking purposes and either prepped for shipping to outside institutions or barcoded (de-identified) and stored in aliquots onsite.
All specimens are retained for sole use of the clinical trial investigation team, as stipulated by the protocol and study principal investigator. Clinical trials specimens are not made available to investigators not associated with the clinical trial, except at the discretion and consent of the study principal investigator.
Services
The MPSR provides support for the understanding of DM/PK/PD and molecular pharmacological mechanisms of new anticancer treatments ranging from new assay and method development, preclinical modeling, protocol development and study design, specimen collection and analysis, data analysis and interpretation.
For assistance, request Molecular Pharmacology Service here (PPMS Access Required):
Signup for a new PPMS account | Quick guide for signing up
NOTICE TO ALL NIH-FUNDED INVESTIGATORS
This resource is funded by the UC Davis Comprehensive Cancer Center Support Grant (CCSG) awarded by the National Cancer Institute (NCI P30CA093373). Publications that have utilized facility resources, services or scientific data generated by the resource should acknowledge the resource or the assistance provided by resource staff and cite the NCI CCSG. An electronic copy of the publication should also be sent to the resource directors.

