Resident Program - Case of the Month
March 2024 – Presented by Dr. Hang Mieu Ha (Mentored by Dr. Morgan Darrow)
Discussion
The upper nasal cavity (including the ethmoid cribriform plate, superior turbinate, and superior half of the nasal septum) is lined by olfactory epithelium which consists of three main cell types: basal cells, olfactory neurosensory cells, and supporting sustentacular cells. Olfactory neuroblastomas are thought to be of neuroectodermal origin and arises from the specialized neurosensory cells found in normal olfactory epithelium.
According to the WHO, olfactory neuroblastoma is an uncommon sinonasal tract tumor, comprising approximately 3% of all intranasal tumors with an estimated incidence of 0.04 cases per 100,000. The tumor may occur at any age from 2-90 years. However, there appears to be a predominance in the fifth through sixth decades of life. Patients often present with nasal obstruction, epistaxis, nasal discharge, and pain. Involvement of adjacent normal structures may present with specific symptoms, as seen in this case with the patient reporting anosmia.
Histologically, this tumor most often demonstrates lobular or sharply demarcated nested architecture separated by fibrovascular septa, although it may also present as solid sheets of cells with vascular stroma. Neoplastic cells are small, round, and blue with high nuclear to cytoplasmic ratio and classic delicate “salt and pepper” neuroendocrine nuclear chromatin. The tumor cells also characteristically display tangled neuronal processes giving a syncytial appearance. Homer Wright pseudorosettes or Flexner-Wintersteiner rosettes may be present as diagnostic clues and when present are helpful for grading purposes. Concretion-like or psammomatous calcifications may be seen as well, as noted in this case. Immunohistochemical studies feature expression of neuroendocrine markers (synaptophysin, chromogranin, CD56) and no staining for cytokeratin. S100 highlights the sustentacular supporting cell framework rimming tumor nests.
Grade as delineated by the Hyams histologic grade system is an important independent prognostic factor. Hyams grading system has four tiers (Grade I to IV) and assesses six histologic features including lobular architecture, amount of fibrillary matrix, mitotic activity, amount of necrosis, nuclear pleomorphism, and the presence of rosette type.
Grade I |
Grade II |
Grade III |
Grade IV |
|
| Architecture | Lobular | Lobular | Variable | Variable |
| Fibrillary matrix | Prominent | Present | Minimal | Absent |
| Mitosis | Absent | Present | Prominent | Marked |
| Necrosis | Absent | Absent | May present | Common |
| Nuclear pleomorphism | Absent | Moderate | Prominent | Marked |
| Rosettes | Homer Wright | Homer Wright | Flexner-Wintersteiner | Flexner-Wintersteiner |
| Hyams grade system (pathology outlines) | ||||
For this case, the absence of necrosis, presence of scattered mitoses, inconspicuous fibrillary matrix, and rare Homer Wright pseudorosettes categorizes the tumor into the Grade II tier.
Staging is another significant prognostic indicator, and although several staging systems exist, none are universally accepted. However, the Kadish system is the most widely used currently and is based on extent of involvement. Confinement to the nasal cavity alone is stage A. Stage B is marked by extension into paranasal sinuses, whereas extension beyond the paranasal sinuses is stage C.
Small cell neuroendocrine carcinoma is a high-grade carcinoma demonstrating overlapping histologic and immunohistochemical features with olfactory neuroblastoma, but is characterized by hyperchromatic nuclear molding, high mitotic rate, and positive cytokeratin staining.
Sinonasal undifferentiated carcinoma (SNUC) is another small blue round cell tumor showing lobular, nested, and solid sheet architecture. However, SNUC is positive for cytokeratin and are characteristically negative for differentiation-specific markers, making this entity a diagnosis of exclusion. SNUC may display staining for neuroendocrine markers but is focal or patchy.
Extranodal NK / T cell lymphoma is an EBV-associated lymphoma most commonly involving the nasal cavity and is therefore an important differential given the mass location. The tumor cells are small to medium sized and may be so confluent as to appear solid sheet like. However, there is often associated pseudoepitheliomatous proliferation and negative staining for CD45 rules out this entity.
Although paragangliomas share a similar immunohistochemical profile with olfactory neuroblastoma (including neuroendocrine marker expression, no cytokeratin, and S100 positive sustentacular cells), paragangliomas are distinguished by characteristic zellballen architecture and neoplastic cells demonstrating much more abundant granular cytoplasm.
References
- WHO Classification of Tumours Editorial Board. "Head and neck tumours" [Internet; beta version ahead of print]. Lyon (France): International Agency for Research on Cancer; 2022 [cited 2024 02 24]. (WHO classification of tumours series, 5th ed.; vol. 9).
- Thompson LD. "Olfactory neuroblastoma". Head Neck Pathol. 2009;3(3):252-259. doi:10.1007/s12105-009-0125-2
- Xu B. "Olfactory neuroblastoma". PathologyOutlines.com website. Accessed February 24th, 2024.
- Jethanamest D, Morris LG, Sikora AG, Kutler DI. "Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors". Arch Otolaryngol Head Neck Surg. 2007;133(3):276-280. doi:10.1001/archotol.133.3.276

 Meet our Residency Program Director
Meet our Residency Program Director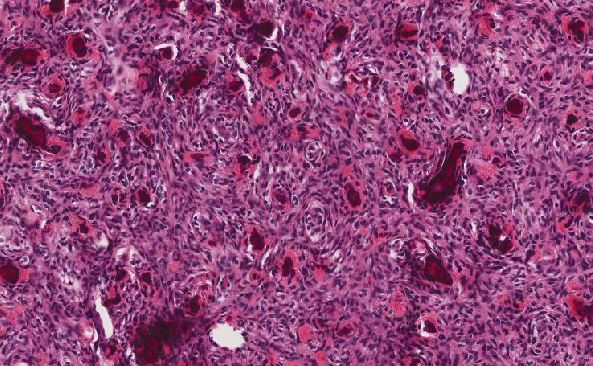
 LeShelle May
LeShelle May Chancellor Gary May
Chancellor Gary May