Clinical trial results suggest potential treatment for recurrent respiratory papillomatosis
Therapy could be the first non-surgical option available for debilitating disease
Patients suffering from the debilitating disease recurrent respiratory papillomatosis (RRP) may soon have hope thanks to the positive results from a clinical trial administered across eight sites including UC Davis Health.
The U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designation to INOVIO Pharmaceuticals for their experimental vaccine INO-3107 as a potential treatment for patients with recurrent respiratory papillomatosis based on data from Phase 1 and 2 of the clinical trial. If approved, INO-3107 could be the first non-surgical treatment available for recurrent respiratory papillomatosis, which is caused by infection with two types of human papillomavirus (HPV).
The FDA's Breakthrough Therapy designation is a process designed to expedite the development and review of promising drugs that would treat a serious condition.
“We are proud to have contributed to this clinical trial to provide an innovative treatment for this complex disease,” said Arnaud F. Bewley, chair of the Department of Otolaryngology - Head and Neck Surgery. “The institutional support we have received has allowed us to create the necessary infrastructure to conduct this type of research, which could potentially save lives and improve the quality of life for many individuals suffering from this disease.”
The Department of Otolaryngology at UC Davis Health is among the top ear, nose and throat programs in the country for care and research. In the most recent U.S. News & World Report’s Best Hospitals survey, the department was the medical center’s highest-ranked specialty – ranking 18th in the nation.
The institutional support we have received has allowed us to create the necessary infrastructure to conduct this type of research, which could potentially save lives and improve the quality of life for many individuals suffering from this disease.”—Arnaud F. Bewley
What is recurrent respiratory papillomatosis?
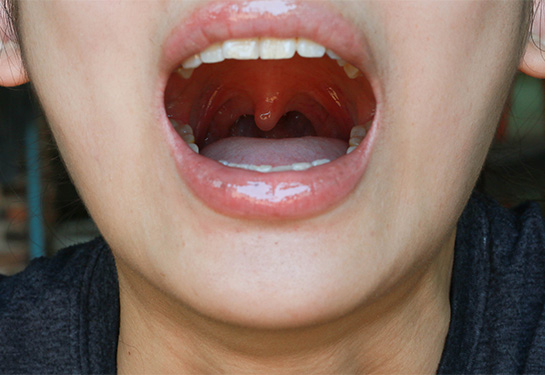
Recurrent respiratory papillomatosis is a debilitating and rare disease caused by infection with HPV types 6 and 11. It characterized by the development of small, wart-like growths, or papillomas, in the respiratory tract. While papillomas are generally benign, they can cause severe, life-threatening airway obstruction and respiratory complications. The disease can also significantly affect quality of life for patients by affecting the voice box, limiting the ability to speak effectively.
Recurrent respiratory papillomatosis is generally broken down into two subtypes: the juvenile-onset form and the adult-onset form. Juvenile cases develop before the age of 12 and are generally more aggressive and recurring.
“Surgery to remove papillomas is the standard of care for recurrent respiratory papillomatosis,” explained Peter C. Belafsky, professor of Otolaryngology and Director of the Center for Voice & Swallowing, who served as the principal investigator for the trial at UC Davis Health. “However, the papillomas often grow back because the underlying HPV infection has not been eradicated, resulting in patients often requiring hundreds of surgical procedures.”
According to the National Organization of Rare Disorders, recurrent respiratory papillomatosis affects roughly 14,000 people in the United States with approximately 1,000 new pediatric cases each year.
Based on the clinical data generated, INO-3107 has the potential to simplify the management of recurrent respiratory papillomatosis and improve the lives of patients living with the disease.”—Peter C. Belafsky
Results from clinical trial
INO-3107 is designed to elicit a targeted T-cell response against HPV-6 and HPV-11, the HPV types that cause RRP. These targeted T cells are designed to seek out and kill infected cells, with the aim of potentially preventing or slowing the growth of new papillomas.
The Breakthrough Therapy designation for INO-3107 was based on data from the phase 1 and 2 open-label trial conducted at UC Davis and eight other sites. Researchers evaluated the safety, efficacy and immune response of the treatment in 32 patients with HPV-6 or HPV-11-associated RRP. The team also looked for any side effects of the treatment.
Study participants had between two and eight surgeries, with four being the average, in the year before receiving INO-3107. Treatment was administered as an intramuscular injection followed by electroporation, a technique in which an electrical field is applied to cells to increase the permeability of the cell membrane, at day 0 and weeks 3, 6, and 9 (4 doses in total).
Overall, 81.3% (26 of 32) patients in the trial had fewer surgeries in the year after receiving INO-3107 compared to the prior year. This included 28.1% (9 of 32) that required no surgical intervention during or after the treatment period.
Data also showed that INO-3107 induced cellular responses against HPV 6 and HPV 11, activating both CD4+ and CD8+ T cells, including cytotoxic CD8 cells; these responses were still observed at week 52. The treatment was well tolerated in the trial, with injection site pain, fatigue, and headache being the most reported adverse events.
“Based on the clinical data generated, INO-3107 has the potential to simplify the management of recurrent respiratory papillomatosis and improve the lives of patients living with the disease,” said Belafsky.