Homing in on a moving target
New mapping strategy designed for lung cancer therapy
Radiation treatments have a simple goal: zap cancer cells until they die. But there’s a downside. While clinicians make every effort to precisely target radiation and hit only tumors, these treatments invariably affect healthy tissues, leading to debilitating, occasionally deadly, side effects. As a result, radiation oncologists must limit doses to spare patients.
This is no small problem. Lung cancer is one of the deadliest malignancies. In 2011, it’s estimated there were more than 220,000 new cases in the United States; around 155,000 of those patients will ultimately die of the disease.
Increasing radiation doses could potentially stem this tide. However, safeguarding healthy tissue is especially difficult when treating lung cancer. As patients breathe, tumors become moving targets.
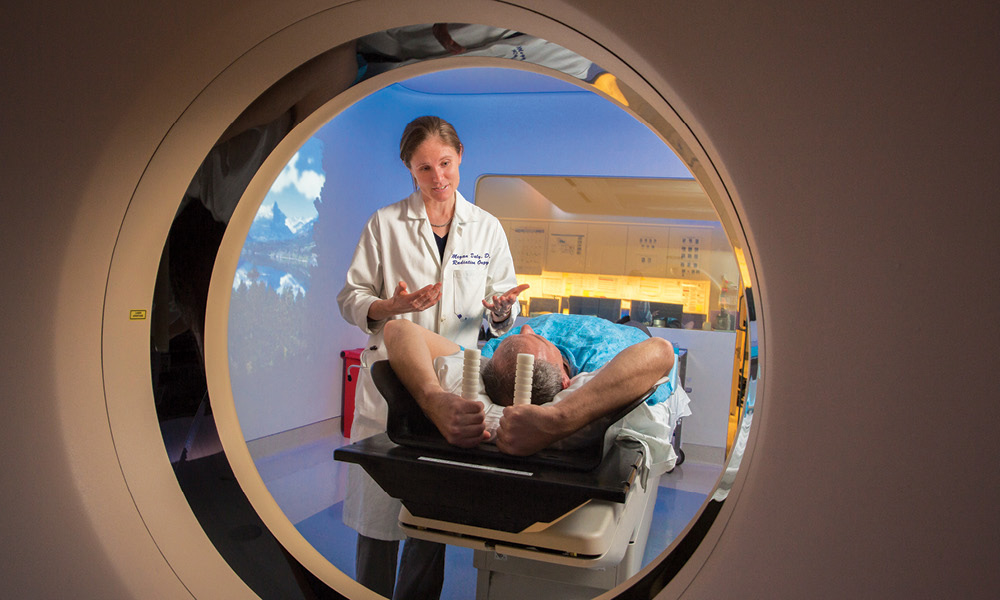
Physicians have been looking for better ways to safely direct radiation at tumors. Now, a group of researchers and clinicians at UC Davis may be close to a solution. The team has developed a unique way to map a patient’s lungs, allowing radiation oncologists to provide even higher doses without compromising patient safety.
A moving target
When radiation was first used against lung cancer decades ago, clinicians lacked the imaging technology to track lung movement and were forced to irradiate tumors along their entire path. This treated the cancer but also damaged the lungs.
Sophisticated imaging systems have made radiation oncology safer and more precise. Now, radiation oncologists use computed tomography (CT) scans before treatment to locate tumors and track their movements.“When a patient gets radiation, there’s a planning session to design the radiation fields,” says Megan Daly, assistant professor in the Department of Radiation Oncology. “We use both a regular CT scan and a 4D scan, which captures the motion of the lungs and the motion of the tumor.”
These scans help the clinical team better target the moving tumor. However, the technology isn’t perfect, and some radiation inevitably hits healthy tissue, generating a number of health risks. A study conducted in 2013 found that 30 percent of patients treated with both chemotherapy and radiation developed serious lung inflammation, a potentially fatal condition called radiation pneumonitis.
“Patients develop pneumonia-like symptoms,” notes Daly. “They have to be treated with weeks of steroids. In the long run, this can reduce overall pulmonary function and exercise capacity, as well as cause lung scarring.”
Next-level precision
Better imaging technologies and software could offer incremental gains in radiation targeting, but there’s another approach that may provide even greater benefits. All lung tissue is not created equal; some areas are more functional, absorbing more life-giving oxygen.
Selectively hitting less-functional regions could increase patient safety. By measuring function in different parts of the lungs, radiation oncologists could map treatment so that any scattered radiation will strike the less active areas, reducing potential side effects, such as pneumonitis.
This new approach is the brainchild of Tokihiro Yamamoto, assistant professor in the Department of Radiation Oncology. Yamamoto has spent more than five years developing ways to measure lung function around tumors, a process called 4D CT ventilation imaging.
“4D CT images provide a movie of a patient’s lung and tumor motion,” says Yamamoto. “Using advanced image-processing technology, we can use these images to estimate which parts of the lung are functioning and which are not. With that information, we can improve radiation treatment planning and delivery to avoid hitting healthy lung regions with high radiation.”
One of the advantages of this technique is that it does not require any additional scans, re-tasking information that’s already being used to plan treatment. Yamamoto and colleagues then apply sophisticated algorithms to measure how patients’ lung volumes change as they inhale and exhale. The resulting data helps him map the most functional areas and miss them during treatment.
“We’re hoping that by avoiding these highly functional lung regions, we will reduce toxicities associated with radiation,” says Yamamoto.
Personalized therapy
The next step for 4D CT ventilation imaging is testing it and designing personalized treatment plans for lung cancer patients. Daly, Yamamoto and colleagues are conducting a small trial to determine whether 4D CT ventilation image-guided radiotherapy is safe.
Each patient will have the functional regions in their lungs mapped so that radiation will preferentially avoid those areas. Although some radiation will still hit normal lung tissue, it will be largely in less-crucial regions. The researchers hope these trials will show this method is safer for patients.
“Inevitably, there is going to be some dose delivered to the lungs,” says Daly. “We’re trying to make sure that dose avoids the most important parts of the lungs.”
While this technology is first being tested for safety, the researchers also will be looking at its effectiveness. Will radiation treatments using 4D CT ventilation imaging do a comparable job at controlling cancer?
If the results are positive, the method could eventually become part of the normal radiation therapy regimen for lung cancer patients. But it’s a long path.
“This is a Phase I trial, testing for safety and feasibility,” says Yamamoto. “If this is successful, we hope to move on to a larger Phase II in the near future.”

