Residency Program - Case of the Month
December 2013 - Presented by Shiloh Martin, M.D.
Answer:
Gastrointestinal stromal tumor (GIST), epithelioid type
Discussion:
Gastrointestinal stromal tumor, or GIST, is the most common mesenchymal tumor of the gastrointestinal (GI) tract and represents 2-3% of all gastric malignancies. They typically arise in older adults with a median age of 55-60 years. GISTs originate from the interstitial cells of Cajal, which are pacemaker cells that regulate peristalsis, located in the myenteric plexus of the GI tract. GISTs can arise anywhere along the GI tract, but most frequently occur in the stomach (50-60%) and small intestine (30-35%), although they can also occur in the colon and rectum (5%) or rarely, the esophagus (<1%). Extra-gastrointestinal tract tumors (E-GISTs) can also arise outside of the GI tract in the abdominal cavity (most often in the omentum, mesentery, or retroperitoneum) and represent <5% of all GISTs. It is thought that a proportion of these E-GISTs may be metastases from an unidentified primary tumor. The malignant potential of GISTs ranges from small benign lesions to aggressive sarcomas. Approximately 10-20% of patients with GISTs present with metastases. Metastases to the liver and omentum are most common, while metastates outside the abdominal cavity are rare.
Three basic morphological patterns are seen in GISTs—spindle cell, epithelioid, and mixed. These patterns overlap with numerous other tumors, such as sarcomas or other mesenchymal neoplasms, sarcomatoid carcinomas, poorly differentiated carcinomas, neural neoplasms, neuroendocrine neoplasms or even metastatic melanoma. This makes immunohistochemistry (IHC) particularly important for confirming the diagnosis. KIT (also known as CD117) and DOG1 (discovered on GIST 1, also known as anoctamin 1 or ANO1) are the two most sensitive and specific markers for GISTs. Approximately 95% of GISTs are positive for KIT/CD117 antigen, an epitope of the KIT receptor tyrosine kinase. However, KIT/CD117 staining can be weakly positive in other mesenchymal neoplasms and thus, KIT/CD117 staining should be interpreted only in the context of other IHC stains, tumor anatomic location, and tumor morphology in order to differentiate GIST from other neoplasms. IHC for DOG1 may help distinguish GIST from other mesenchymal tumors, particularly those that are KIT-negative. DOG1 is a protein of unknown function that is expressed strongly on GIST and is rarely expressed on other soft tissue tumors.
Most GISTs (95%) are sporadic with no established risk factors. Approximately 75-80% of all GISTs contain a mutation in the KIT receptor that results in constitutive activation of the protein. Mutations in five KIT exons have been observed in GIST: exon 11 (67%), exon 9 (10%), and exons 8, 13, and 17 (3%). GISTs are usually heterozygous for a particular mutation, but loss of the remaining wild-type KIT allele occurs in 8-15% of tumors and may be associated with malignant progression. KIT-mutant tumors typically still express DOG1. 20-25% of GISTs do not have KIT mutations, and of these tumors, approximately one-third have mutations in PDGFRA (platelet-derived growth factor receptor alpha), a close homolog of KIT with similar extracellular and cytoplasmic domains. KIT and PDGFRA mutations are mutually exclusive. PDGFRA-mutant GISTs tend to exhibit a predilection for the stomach, epithelioid morphology, nuclear pleomorphism, and variable expression of KIT/CD117. As with KIT-mutant GISTs, PDGFRA-mutant GISTs express DOG1. A small proportion of GISTs (5%) are completely negative for KIT/CD117 via IHC. It is thought that in these cases, IHC lacks sufficient sensitivity to detect small amounts of the mutant kinase—about one-third of these tumors have PDGFRA mutations, but more than half turn out to have KIT mutations by genotyping. In approximately 12-15% of GISTs, no detectable mutations in KIT or PDGFRA are identified. In these so-called wild-type GISTs, KIT is still phosphorylated (constitutively active) despite the lack of a detectable gene mutation.
The non-sporadic GISTs (5% of GISTs) are associated with specific tumor syndromes. A subset of patients with neurofibromatosis type 1 (von Recklinghausen’s neurofibromatosis) will develop one or more GISTs, mainly in the small intestine. These tumors are strongly KIT positive by IHC, yet are often negative for any KIT mutations. Familial GIST syndrome patients typically have mutations in KIT exons 8, 11, 13, or 17 and are at high risk for developing one or more GISTs at a young age, usually in the stomach or small intestine. A smaller subset of patients with familial GIST syndrome may have germline PDGFRA exon 12 mutations. Carney’s triad (gastric GIST, paraganglioma, and pulmonary condroma) is a rare, non-heritable syndrome seen mainly in young women. These tumors usually do not have KIT or PDGFRA mutations, but have a loss of succinate dehydrogenase subunit B (SDHB) protein expression, though no mutations in genes encoding succinate dehydrogenase subunits have been reported. Patients with Carney-Stratakis syndrome (GIST and paraganglioma) on the other hand, do have germline mutations in succinate dehydrogenase subunit genes (SDHA, SDHB, SDHC, and SDHD).
The treatment of GISTs usually involves complete surgical excision. However, the proper identification of GISTs with genotyping is critical because of the availability of specific, molecular-targeted therapy, which may be important particularly for those patients with unresectable tumors or metastatic disease. Imatinib mesylate (Gleevec), a tyrosine kinase inhibitor, is effective in most cases of GIST with KIT mutations and is also effective in many cases with PDGFRA mutations. GISTs lacking KIT or PDGFRA mutations most likely will be unresponsive to such therapy. Mutational analysis is useful for treatment planning because different mutations of KIT and PDGFRA may respond differently to therapy. It is also important to note that secondary GIST mutations can be acquired during therapy. In such cases, the patient usually presents with metastatic disease with acquired drug resistance, and this is the result of secondary, imatinib-resistant KIT or PDGFRA mutations arising during imatinib treatment.
References:
Joensuu H, Hohenberger P, Corless CL: Gastrointestinal stromal tumor. Lancet 382: 973-83, 2013.
Cellular and Molecular Classifications of Gastrointestinal Stromal Tumors. http://www.cancer.gov/cancertopics/pdq/treatment/gist/HealthProfessional/page2
Corless CL, Heinrich MC: Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 3: 557-86, 2008.
http://surgpathcriteria.stanford.edu/gitumors/gist-gastrointestinal-stromal-tumor/
Heinrich MC, Corless CL, Demetri GD, et al.: Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21: 4342-9, 2003.
Miettinen M, Lasota J: Histopathology of Gastrointestinal stromal tumor. J Surg Oncol 104: 865-73, 2011.

 Meet our Residency Program Director
Meet our Residency Program Director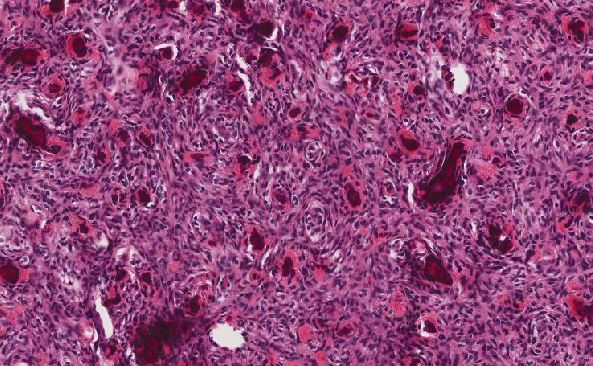
 LeShelle May
LeShelle May Chancellor Gary May
Chancellor Gary May