Case of the Month
April 2016 - Presented by Dr. Andrew Jones & Dr. Karen Matsukuma
Answer:
C: Combined neuroendocrine carcinoma (G3) and tubulovillous adenoma
Diagnosis:
The correct diagnosis is combined neuroendocrine carcinoma (G3) and tubulovillous adenoma. The GI neuroendocrine system is the largest endocrine system in the body, producing more than 30 hormones that help regulate motility, digestion, and immune surveillance within the gut. Neuroendocrine neoplasms of the GI tract are rare, occurring 1-2 per 100,000 people; they constitute only 2% of all GI tumors. However, they constitute approximately 75% of all neuroendocrine neoplasms that occur in the body.
The most common sites for neuroendocrine neoplasms are the ileum, appendix, and rectum; tumors of the esophagus are very rare. Patients can have non-specific symptoms, including abdominal pain, nausea, diarrhea, and weight loss, though approximately 50% are discovered incidentally (usually either at appendectomy or autopsy). Only 5-10% of GI neuroendocrine neoplasms are associated with the clinical carcinoid syndrome (i.e. flushing, diarrhea, asthma, tricuspid regurgitation), as this requires a large and functional tumor to overcome hepatic first pass metabolism of secreted hormones (or a tumor that has metastasized to the liver).
The etiology of neuroendocrine neoplasms is still a matter of controversy and research, but recent studies suggest that gut endocrine cells originate from the endoderm (not the neuroectoderm), and are capable of multidirectional differentiation.
The classification and nomenclature of neuroendocrine neoplasms of the gut (and throughout the body) has been the subject of much debate and modification. The 4th edition of the WHO Classification of Tumors of the Digestive System (published in 2010) has established the following guidelines:
| Name | Past names | Defining characteristic |
| Neuroendocrine tumor G1 (Grade 1) | Carcinoid, well-differentiated endocrine tumor | Mitotic count <2 per 10 high power fields and/or ≤2% Ki67 index |
| Neuroendocrine tumor G2 (Grade 2) | Well-differentiated endocrine carcinoma | Mitotic count 2-20 per 10 high power fields and/or 3-20% Ki67 index |
| Neuroendocrine carcinoma | Small cell carcinoma, large cell neuroendocrine carcinoma, poorly differentiated neuroendocrine carcinoma | Mitotic count >20 per 10 high power fields and/or >20% Ki67 index |
| Mixed adenoneuroendocrine carcinoma (MANEC) | Mucocarcinoid, mixed forms carcinoid adenomcarinoma, mixed exocrine-endocrine carcinoma (MEEC) | Adenocarcinoma and neuroendocrine carcinoma |
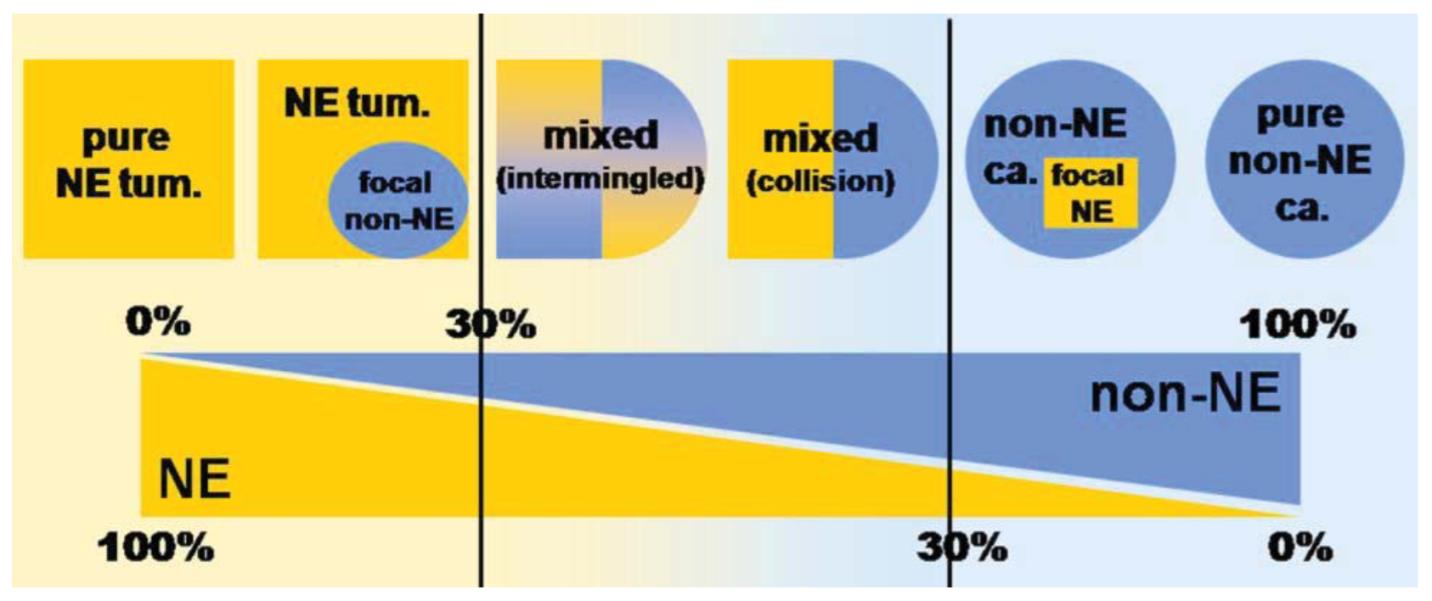
This case shows evidence of a tubulovillous adenoma with an associated neuroendocrine carcinoma, evidenced by the synaptophysin and CAM5.2 positivity and Ki67 proliferative index of > 20%. Up to 21 mitotic figures were also noted in 10 high power fields. This lesion does not meet the criteria for MANEC, as the glandular component is not malignant (i.e., adenocarcinoma). Tumors classified as MANECs, in addition to having carcinomatous elements of glandular and neuroendocrine differentiation, must be composed of at least 30% of each type. The prognosis of MANECs is typically thought to follow that of the more aggressive lesion (i.e., neuroendocrine carcinoma). Tumors with both adenocarcinoma and neuroendocrine carcinoma (but less than 30% of one component) can be described descriptively as adenocarcinoma with focal neuroendocrine differentiation or neuroendocrine carcinoma with focal glandular differentiation (Figure 7).
Figure 7: MANECs of the GI tract must show at least 30% NE and non-NE components.
Image from La Rosa, et al.
There is evidence linking colorectal NETs and NECs with ulcerative colitis and Crohn disease. Patients with colorectal NETs and NECs are at increased risk of other malignancies; metachronous or synchronous non-neuroendocrine neoplasms are found in approximately 13% of patients. Studies have suggested that the incidence of high grade or villous dysplasia overlying both low- and high-grade colonic neuroendocrine neoplasms is significantly higher than in adenomas without neuroendocrine neoplasms.
Rectal neuroendocrine neoplasms account for 1-2% of all rectal tumors and 17% of GI neuroendocrine neoplasms. They have an approximately equal gender incidence and most often occur in the sixth decade of life. The incidence of these neoplasms has doubled from 1950 to 1991, and they are particularly prevalent in Asians / Pacific Islanders, Native Americans, and African Americans. They are almost exclusively derived from hindgut L-cells and are typically 1cm or less in size and located in the distal rectum. They usually express synaptophysin but may not express chromogranin A.
In any patient with a new diagnosis of large or small cell NEC, clinical evaluation for an alternative primary site must be performed, as these tumors are morphologically identical to neuroendocrine neoplasms of other body sites and thus may represent metastatic spread. In this case, the presence of a pre-malignant or in situ (e.g., adenomatous) component in association with the neuroendocrine carcinoma supports the conclusion that this is the primary site.
Works Cited
Bosman, F. T. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer, 2010. Print.
Estrella, Jeannelyn S., Melissa W. Taggart, Asif Rashid, and Susan C. Abraham. "Low-grade Neuroendocrine Tumors Arising in Intestinal Adenomas: Evidence for Alterations in the Adenomatous Polyposis Coli/β-catenin Pathway." Human Pathology 45.10 (2014): 2051-058. Web. 16 Apr. 2016.
Eze, Ogechukwu, Saul Harari, Margaret Cho, and Antonio Galvao Neto. "Colonic Polyp Presenting as a Tubulovillous Adenoma and Harbinger of High-grade Neuroendocrine Carcinoma: A Unique Presentation."Case Reports in Clinical Pathology 2.1 (2015): 67-71. Sciedu Press, 24 Oct. 2014. Web. 16 Apr. 2016.
Ihtiyar, Enver. "Small Cell Carcinoma of Rectum: A Case Report." World Journal of Gastroenterology WJG 11.20 (2005): 3156. Web.
Joshua, AM, D. Adams, P. McKenzie, M. Solomon, and SJ Clarke. "Case 2: Small-Cell Carcinoma of the Rectum." Journal of Clinical Oncology (2005): 912-13. Web.
La Rosa, Stefano, Alessandro Marando, Fausto Sessa, and Carlo Capella. "Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update." Cancers 4.4 (2012): 11-30. Web.
Lee, So-Young, Dae-Yong Hwang, Tae Sook Hwang, Wan-Seop Kim, So Dug Lim, Wook Youn Kim, Se-Hun Kim, and Hye Seung Han. "Neuroendocrine Dysplasia Combined in a Tubular Adenoma of Rectum: A Case Report." Korean Journal of Pathology Korean J Pathol 47.5 (2013): 495. Web.
Lee, Sun Mi, Soomin Ahn, Yun Kyung Lee, Ki Taek Jang, Cheol Keun Park, and Kyoung-Mee Kim. "Neuroendocrine Tumor in Gastric Adenoma: A Diagnostic Pitfall Mimicking Invasive Adenocarcinoma."Diagnostic Pathology Diagn Pathol 7.1 (2012): 102. Web.
Lin, Jingmei, John R. Goldblum, Ana E. Bennett, Mary P. Bronner, and Xiuli Liu. "Composite Intestinal Adenoma-Microcarcinoid." American Journal of Surgical Pathology 36.2 (2012): 292-95. Feb. 2012. Web. 16 Apr. 2016.
Lipka, Seth. "Synchronous Small Cell Neuroendocrine Carcinoma and Adenocarcinoma of the Colon: A Link for Common Stem Cell Origin?" ACGCR ACG Case Reports Journal 1.2 (2014): n. pag. Web.
Odze, Robert D., John R. Goldblum, and James M. Crawford. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. Philadelphia, PA: Saunders, 2004. Print.
Qasem, E., M. Nangalia, H. Jones, P. Griffiths, C. Sekaran, MD Evans, M. Davies, U. Khot, J. Beynon, N. Williams, and DA Harris. "Small Cell Carcinoma of the Rectum, A Systematic Literature Review and Case Series." Colorectal Cancer: Open Access 2.1:10 (2016): n. pag. IMedPub Journals, 8 Jan. 2016. Web. 16 Apr. 2016.
Sarsfield, P., and PP Anthony. "Small Cell Undifferentiated ('neuroendocrine') Carcinoma of the Colon." Histopathology 16 (1990): 357-63. Web.
Vilor, Mabel, Yutaka Tsutsumi, R. Yoshiyuki Osamura, Nobuhiro Tokunaga, Jin-Ichi Soeda, Masatoshi Ohta, Hisao Nakazaki, Yasuhisa Shibayama, and Fumiake Ueno. "Small Cell Neuroendocrine Carcinoma of the Rectum." Pathology International 45.8 (1995): 605-09. Web. 16 Apr. 2016.

 Meet our Residency Program Director
Meet our Residency Program Director
 LeShelle May
LeShelle May Chancellor Gary May
Chancellor Gary May