March 2015
March 2015 - Presented by Dr. R. Jeanna Su & Dr. Karen Matsukuma
Answer:
- Small bowel adenocarcinoma, moderately to poorly differentiated
- Gastrointestinal ganglioneuromatosis
- Neurofibromatosis type 1 (NF-1) vasculopathy
- Gastrointestinal stromal tumor (GIST)
Discussion:
Primary neoplasms of the small intestine are very rare, comprising approximately 4% of all tumors of the gastrointestinal tract. Of those, approximately 60% are malignant. Presenting signs and symptoms are non-specific and include anemia, gastrointestinal bleeding, abdominal pain, and small bowel obstruction.
The most frequent malignant tumors of small bowel are: adenocarcinoma, lymphoma, well-differentiated neuroendocrine tumor (formerly termed carcinoid), and gastrointestinal stromal tumor (GIST). Although the majority of primary small bowel carcinomas are sporadic, a significant number are associated with defined syndromes and diseases, including neurofibromatosis type 1 (NF-1), also known as von Recklinghausen disease.
Four categories of gastrointestinal manifestations have been reported in NF-1 patients: 1. true neurogenic neoplasms, e.g. neurofibroma, ganglioneuromatosis, malignant peripheral nerve sheath tumor; 2. gastrointestinal stromal tumor (GIST); 3. neuroendocrine neoplasms; and 4. miscellaneous tumors and lesions, including adenocarcinoma and vasculopathy.
In the current case, microscopic findings demonstrate a neoplasm composed of well-formed glands in some areas and solid sheets of neoplastic cells in other areas, compatible with a moderately to poorly differentiated adenocarcinoma. At least focally, residual adenoma with high grade dysplasia was present, establishing this as the primary site of neoplasia. The background mucosa also demonstrates a diffusely S100 positive spindle cell proliferation with scattered ganglion cells. No significant cytologic atypia, enhanced cellularity, or mitotic activity is identified to suggest a malignant peripheral nerve sheath tumor. The presence of a benign neural or nerve sheath tumor with admixed ganglion cells is consistent with ganglioneuroma. In this case, the expansile nature of the lesion is in keeping with intestinal ganglioneuromatosis.
In the mesentery, scattered medium-sized arteries demonstrate marked intimal thickening. Although more often recognized in other sites such as the kidney and aorta, NF-1 vasculopathy has been well-documented in the gastrointestinal tract. NF-1 vasculopathy is a less well known phenomenon in the setting of neurofibromatosis; however, it should be kept in mind as it can be the cause of myriad complications in the NF-1 patient.
On histologic sections, the 2 cm serosal nodule demonstrates a well-circumscribed neoplasm composed of spindle cells arranged in vague fascicles. The morphology along with the diffuse immunopositivity for CD117 and negativity for S100 and desmin is diagnostic of GIST.
Although GIST is a well-known gastrointestinal manifestation of NF-1, its clinical significance in identifying NF-1 patients is eclipsed by the overwhelming number of sporadic cases. However, specific combinations of gastrointestinal manifestations, such as co-existence of GIST with duodenal neuroendocrine tumor and/or gastrointestinal peripheral nerve sheath tumor is far less common, leading some observers to suggest that this is an almost pathognomonic feature of NF-1. Review of the literature also reveals several reports of NF-1 patients with small bowel adenocarcinoma, along with co-existent GIST and gastrointestinal peripheral nerve sheath tumors. Due to the relatively rarity of sporadic primary small bowel adenocarcinoma, it seems likely these adenocarcinomas are related to NF-1 mutation as well. Thus, the combination of GIST with gastrointestinal neuroendocrine tumor, peripheral nerve sheath tumor, and/or small bowel adenocarcinoma should prompt the clinician and/or pathologist to evaluate for the possibility of NF-1 (if this information is not already known).
References:
Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen’s disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012; 5(9): 852–862.
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Reotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Human Pathology 2002; 33:459.
Jones TJ and Marshall TL. Neurofibromatosis and small bowel adenocarcinoma: an
unrecognised association. Gut 1987, 28:1173.
Behranwala KA, Spalding D, Wotherspoon A, Fisher C, and Thompson JN. Small bowel gastrointestinal stromal tumours and ampullary cancer in Type 1 neurofibromatosis. World Journal of Surgical Oncology 2004; 2: 1.
Lie, JT. Vasculopathies of Neurofibromatosis Type 1 (von Recklinghausen Disease). Review. Cardiovascular Pathology. 1998 Mar-Apr; 7(2): 97.
Stratopoulos C, Papakonstantinou A, Anagnostopoulos G, Terzis I, Tzimas G, Gourgiotis S, Vamvouka C, Hadjiyannakis E. Intestinal neurofibromatosis and small-bowel adenocarcinoma: a single case study. European Journal of Cancer Care. 2009 Sep;18(5):466.
Tewari N, Rollins K, Gandhi N, Kaye P, Lobo DN. Mixed periampullary adenocarcinoma and somatostatinoma with small bowel gastrointestinal stromal tumour in neurofibromatosis type I Journal of the Pancreas 2014 Nov 28;15(6):600.

 Meet our Residency Program Director
Meet our Residency Program Director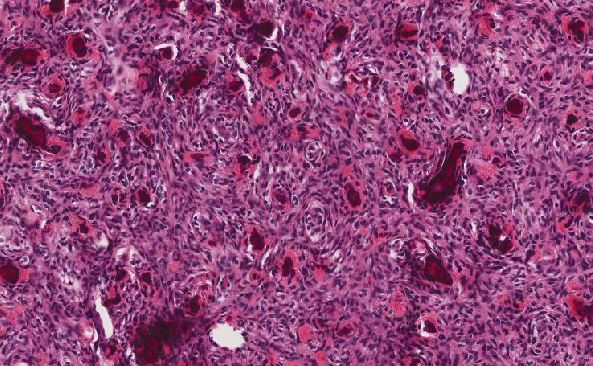
 LeShelle May
LeShelle May Chancellor Gary May
Chancellor Gary May