Residency Program - Case of the Month
February 2010 Final Diagnosis - Presented by Mary Tomic, M.D.
Answer:
Mesonephric adenocarcinoma
Histologic description:
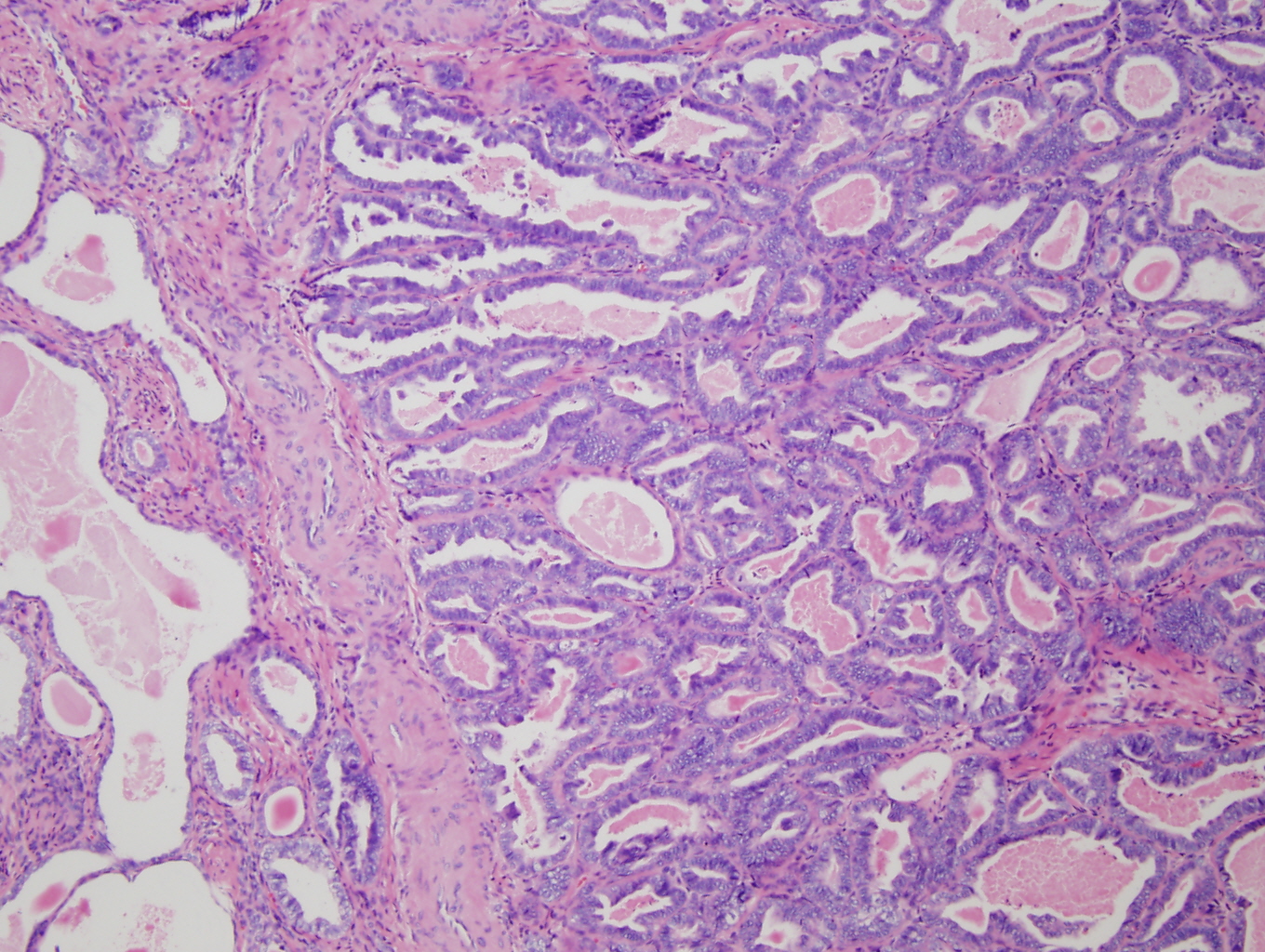
Microscopically the tumor involved the lower uterine segment and the entire cervix. Depth of extension was 95 – 100% of the cervical wall thickness and the paracervical soft tissue was positive for tumor. The tumor showed a mixed appearance with: areas of crowded round to irregular glands, some with papillary projections and some with eosinophillic intraluminal secretions (Figure 1-3); areas of small round tubules (Figure 4); and areas of scattered cystically dilated ducts with dense eosinophillic intraluminal secretions (Figures 2,3). These structures were lined by one to multiple layers of columnar to cuboidal to flattened cells. The cells themselves were mildly pleomorphic, had scant eosinophillic cytoplasm and hyperchromatic nuclei with somewhat coarse chromatin and occasional, single indistinct nucleoli. Mitotic figures were rare. There was no desmoplastic stromal response.
Discussion:
Mesonephric adenocarcinoma is a malignant neoplasm that arises from mesonephric remnants. The mesonephric (Wolffian) duct is one of the paired embryogenic tubules (along with the Mullerian duct) that drain the primitive kidney (mesonephros) to the cloaca. In both males and females the mesonephic duct gives rise to the trigone of the bladder. In the presence of testosterone the mesonephric duct develops into the rete testis, efferent ducts, epididymes, seminal vesicles and vasa deferentia. In the absence of testosterone the mesonephric duct regresses. Remnants may persist however as the epoopheron, Skenes glands, and Gartner's duct, and may subsequently give rise to cysts and neoplasms (1).
Mesonephric adenocarcinoma is rare; thirty three cases arising in the uterine cervix, four cases arising in the uterine corpus and four cases arising in the vagina have been reported in the literature (2-13).
Previous reports of mesonephric adenocarcinoma have described five morphologic patterns with most tumors displaying more than one pattern (2-5, 7-13). These patterns include: ductal, retiform, tubular, solid, and sex-cord like. The ductal pattern consists of varying sized tubular glands which may have intraglandular papillae, and are lined by single to multiple layers of columnar cells. The retiform pattern shows irregular slit-like branching tubules with intraluminal papillae; the tubules and papillae are lined by one to many layers of columnar to cuboidal to flattened cells. The tubular pattern is characterized by small, round, back to back tubules lined by cuboidal to flattened cells. The solid pattern consists of solid sheets of cells punctuated by scattered glands. Intraluminal eosinophillic, hyaline secretions may be seen in all of these patterns. Cords and trabeculae of cells with minimal eosinophillic cytoplasm characterize the sex-cord stromal pattern. All patterns display nuclear hyperchromasia and mild to moderate nuclear pleomorphism with occasional small, single nucleoli. Coarse to vesicular nuclear chromatin has been reported in the ductal pattern (4). Mitotic rates have been reported to range from 3 to 50 per HPF with the highest mitotic rates present in areas with ductal and solid growth patterns (3, 4). The surrounding stroma does not display a desmoplastic response. Finally mesonephric hyperplasia is typically seen adjacent to or in the background of malignant mesonephric neoplasia (2-4). Please see Clement et al. 1995 for the original descriptions and photographs of these patterns (8).
Silver et al. (2001) reported the largest series of cases (11) of mesonephric adenocarcinoma with the most comprehensive description of tumor immunophenotype. All of the tumors they studied were negative for CK20, ER, PR, and CEA; all were positive for AE1/AE3, CAM5.2, EMA, and CK7; 88% were positive for calretinin and 70% were positive for vimentin. Ordi et al. (2003) performed immunohistochemical analysis for CD10 expression on 417 cases of neoplastic, hyperplastic and normal gynecologic specimens (6). They found ubiquitous expression of CD10 in mesonephric remnants and mesonephric tumors and absent CD10 expression in neoplastic and non neoplastic mullerian epithelia. CD10 was positive in 7 of the 13 subsequently reported cases of mesonephric adenocarcinoma (8 – 13).
The differential diagnosis for this tumor varies with the morphologic patterns exhibited by it. The ductal pattern resembles adenoma malignum and the villoglandular variant of endemetroid carcinoma. In fact, an endometrial biopsy was performed in the current case and was interpreted as FIGO grade 1 endometrial adenocarcinoma with villoglandular features. Figure 1 shows an area of tumor that exhibits the ductal pattern with tubular structures with intraglandular villous papillae. As previously stated however, the tumor exhibited other morphologic patterns. The presence of varied morphology is an important clue for the diagnosis of mesonephric adenocarcinoma as these tumors typically display more than one morphologic pattern. Further, mesonephric hyperplasia is often simultaneously present (although it was not present in the current case) and mesonephric carcinomas are negative for ER and PR whereas most well differentiated endometrial adenocarcinomas express these antigens. Please see Table 1 for a comparison of the immunophenotypes of mesonephric, endocervical, and endometrial adenocarcinomas.
| Immunostain | Percentage of Tumors with Positive Staining | ||
| Mesonephric Adenocarcinoma | Endocervical Adenocarcinoma | Endometrial Adenocarcinoma | |
| Pancytokeratin | 100% | 100% | 100% |
| CK7 | PR | 100% | 92% |
| CK20 | 0% | 3% | 4% |
| CD10 | 82% | 9% | 3% |
| Calretinin | 76% | 5% | 6% |
| EMA | 93% | 92% | 98% |
| CEA | 0% | 86% | 10% |
| ER | <1% | 21% | 75% |
| PR | <1% | 21% | 69% |
Data for immunostaining characteristics of mesonephric carcinoma derived from references 2-3, 4-9, 11-13. Data for immunostaining characteristics of endocervical (except calretinin – reference 14) and endometrial adenocarcinomas derived from Immunoquery.
Tumors with a predominately tubular pattern must be distinguished from mesonephric hyperplasia. Features supportive of malignancy include loss of intervening stroma between glands and nuclear atypia; the presence of lymphovascular or perineural invasion confirms it. Retiform and sex-cord stromal patterns must be differentiated from uterine tumors resembling ovarian sex cord stromal tumors (UTROCTs). Again the presence of mesonephric hyperplasia suggests mesonephric adenocarcinoma. Also, UTROCTs rarely occur in the cervix whereas the majority of reported cases of mesonephric adenocarcinoma have involved the cervix. Finally mesonephric adenocarcinomas do not exhibit smooth muscle or endometrial stromal differentiation.
Of the cases for which clinical follow up is available (27 cases), 7 patients died of disease within 0.8 – 9 years; 20 patients were alive at latest follow-up (1.5 – 10 years); and fifteen patients experienced recurrent disease (2-4, 7, 10). Due to the rarity of this disease optimal treatment has not been definitively established; certainly a minimum of complete surgical excision is required.
References:
- Moore KL, Persaud TVN. The urogenital system. In: The developing human: clinically oriented embrology. Philladelphia: WB Saunders, 2002.
- Ferry JA, Scully RE. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix: a study of 49 cases. American journal of surgical pathology 1990; 14(12): 1100-1111.
- Clement PB, Young RH, Keh P, Ostor A, Scully RE. Malignant mesonephric neoplasms of the uterine cervix: a report if eight cases, including four with a malignant spindle cell component. American journal of surgical pathology 1995; 19(10): 1158-1171.
- Silver SA, Devouasspux-Shisheboran M, Mezzetti TP, Tavassoli FA. Mesonephric adenocarcinomas of the uterine cervix: astudy of eleven cases. American journal of surgical pathology 2001; 25(3): 379-387.
- Ordi J, Nogales FF, Palacin A, Marquez M, Pahisa J, Vanrell JA, Cardesa A. Mesonephric carcinoma of the uterine corpus: CD10 expression as evidence of mesonephric differentiation. American journal of surgical pathology 2001; 25(12): 1540-1545.
- Ordi J, Romagosa C, Tavasosoli FA, Nogales F, Palacin A, Condom E, Torne A, Cardesa A. CD10 expression in epithelial tissues and tumors of the gynecologic tract: a useful marker in the diagnosis of mesonephric, trophoblastic, and clear cell tumors. American journal of surgical pathology 2003; 27(2): 178-186.
- Bague S, Rodriguez IM, Prat J. Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of nine cases. American journal of surgical pathology 2004; 28(5): 601-607.
- Angeles RM, August CZ, Weisenberg E. Pathologic quiz case: an inicidentally detected mass of the uterine cervix. Archives of pathology and laboratory medicine 2004; 128: 1179-1180.
- Ersahin C, Huang M, Potkul RK, Hammadeh R, Salhadar A. Mesonephric carcinoma of the vagina with 3 year follow up. Gynecologic oncology 2005; 99: 757-760.
- Marquette A, Moarman P, Vergote I, Amant F. Second case of uterine mesonephric adenocarcinoma. International journal of gynecological cancer 2006; 16: 1439-1478.
- Bifulco G, Mandato VD, Mignogna C, Giampalolino P, Sardo A, De Cecio R, De Rosa G, Piccoli R, Radice L, Nappi C. A case of mesonephric carcinoma of the vagina with a 1-year follow up. International journal of gynecological cancer 2007; 18: 1108-1131.
- Fukunga M, Takahasi H, Yasuda M. Mesonephric carcinoma of the uterine cervix: a case report with immunohistochemical and ultrastructural studies. Pathology research and practice 2008; 204: 671-676.
- Wani Y, Notohara K, Tsukayama C. Mesonephric adenocarcinoma of the uterine corpus: a case report and review of the literature. International journal of gynecological cancer 2008; 27: 346-352.
- McCluggage WG, Olivia E, Herrington CS, McBride H, Young RH. CD10 and calretinin staining of endocervical glandular lesions, endocervical stroma and endometroid adenocarcinomas of the uterine corpus: CD10 positivity is charctersitic of, but not specific for, mesonephric lesions and is not specific for endometrial stroma. Histopathology 2003; 43: 144-150.
|
Figure 1: Low power (4X) view of section of tumor from the lower uterine segment. The tumor displays the ductal pattern of mesonephric adenocarcinoma with intraglandular villous papillae. Note the similarity of this appearance to the villoglandular variant of endometroid endometrial adenocarcinoma. secreting cells and containing papillary intracystic projections. |
Figure 2: Low power (4X) view of tumor from lower uterine segment and cervix. A focus of irregular angular glands (some with papillary projections) is present adjacent to a varied proliferation of small and cystic dilated tubules (many of which have dense eosinophillic intraluminal secretions). |
|
Figure 3: Mid-power (10X) view of tumor from cervix showing angulated glands adjacent to scattered cystically dilated and small tubules. These structures are lined by columnar to cuboidal to flattened cells with hyperchromatic nuclei. Dense, eosinophillic intraluminal secretions are a prominent feature. |
Figure 4: High power (20X) view of tumor from cervix showing tubular pattern with crowded small round tubules lined by single to multiple layers of cuboidal to low columnar cells with enlarged hyperchromatic nuclei. |
|
|
|
|
|




 Meet our Residency Program Director
Meet our Residency Program Director
 LeShelle May
LeShelle May Chancellor Gary May
Chancellor Gary May