Join Us in Making a Difference
You can help improve diagnostic testing and identify better treatments for neurodevelopmental disabilities.
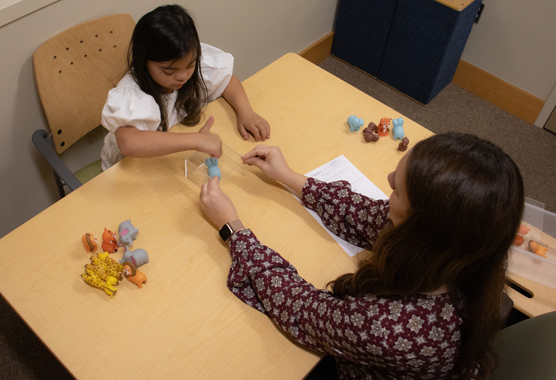
The MIND Institute has research studies focusing on autism, Down syndrome, fragile X syndrome, attention-deficit/hyperactivity disorder (ADHD), and chromosome 22q11.2 deletion syndrome. Our researchers explore everything from fundamental biological research to clinical trials for treatments, leading to new insights into the genetic and environmental causes of neurodevelopmental disabilities. Their work also involves identifying differences in brain structure and function, pioneering interventions, and advancements in very early diagnosis.
You can play a significant role in helping researchers better understand neurodevelopmental disabilities that affect many people. Your participation can help improve diagnostic testing and identify better treatments.
-
Angkustsiri Lab
Learn moreDevelopmental-behavioral pediatrician Kathy Angkustsiri leads a team that studies the developmental and behavioral characteristics of various neurodevelopmental disabilities. These include chromosome 22q11.2 deletion syndrome, Down syndrome and autism. Current studies include evaluating the use of stimulant medication in children with Down syndrome who also have ADHD.
-
Autism Phenome Project
Learn moreThe goal of the Autism Phenome Project is to identify and describe different types of autism. The project aims to identify groups of children with common traits, things like shared patterns of brain development or similar behaviors.
-
Attention, Impulsivity and Regulation
Learn moreThis program works to improve the lives of children, teens and adults experiencing challenges with attention, impulsivity, or cognitive and emotional regulation. This includes helping people with attention deficit/hyperactivity disorder (ADHD), vulnerability for substance use and problems with self-regulation. Areas of research include brain function and development in ADHD, virtual reality treatment for improving attention and testing links between fidgeting and attention. The program is directed by Julie Schweitzer, professor in the Department of Psychiatry and Behavioral Sciences. The program’s clinic director is licensed clinical psychologist J. Faye Dixon.
-
Collaborative START Lab
Learn moreThe Collaborative START lab aims to improve the quality of life of autistic people and their families by improving access to high quality education and treatment through translation of evidence-based treatment into effective, publicly funded community services that reach traditionally underserved populations.
-
Early Detection Lab
Learn moreThis lab, led by Professor of Psychiatry and Behavioral Sciences Sally Ozonoff, studies how to identify autism as early in childhood as possible. Early identification of autism helps children get services when they are most beneficial. Our current work explores the use of telehealth assessments and online screeners to improve early detection and access to diagnostic services for families in underserved communities.
-
Fragile X Research and Treatment Program
Learn moreThis program is directed by Randi Hagerman, developmental-behavioral pediatrician and world-renowened fragile X researcher, and provides comprehensive clinical services, including psychological testing and medical assessment. The multidisciplinary program includes specialist in pediatrics, molecular genetics, psychiatry, psychology, neurology, neurobiology, pathology and social work. Researchers conduct a wide variety of studies that advance understanding of fragile X-related contitions and treatment.
-
Laboratory on Language Development in Neurodevelopmental Disabilities
Learn moreThis research lab, led by former MIND Institute director Leonard Abbeduto, is trying to understand how language develops in children who have certain developmental disabilities like fragile X syndrome, Down syndrome and autism. Researchers have noticed that each condition has its own unique characteristics when it comes to language, with different areas where children may do well or struggle.
-
Miller Lab
Learn moreLed by Associate Professor of Psychiatry and Behavioral Sciences Meghan Miller, the goal of this lab is to uncover early signs of autism and ADHD in infants and toddlers, and to better understand the links between these two conditions. They closely track the development of infants and toddlers to gain a better understanding of their attention skills, emotion regulation, self-control, and social and communication skills over the first few years of life.
-
ReCHARGE and ECHO Studies
Learn moreBy participating in or studies, your family will contribute to a better understanding of how our environment can be improved in schools, communities, and homes to improve children’s lives: their achievements, their relationships with others, and their health.
-
Solomon Lab
Learn moreThis lab, led by MIND Institute Interim Director Marjorie Solomon, uses cognitive neuroscience methods to advance the study of higher cognition in autism. One aspect of this research focuses on cognitive control — the ability to flexibly allocate mental resources to guide thoughts and actions in light of internal goals. Researchers use neuroimaging to study brain activity and patterns from adolescence through young adulthood. The Solomon lab also studies learning memory in this same age group.
-
Translational Psychophysiology and Assessment Laboratory
Learn moreThis lab, called T-PAL (Translational Psychophysiology and Assessment Laboratory), focuses on understanding how genes, the brain, and behavior are connected in neurodevelopmental disabilities, particularly in conditions related to fragile X syndrome. Current studies include long-term research into possible biomarkers for FXTAS (fragile X-associated tremor/ataxia syndrome) and the investigation of a smartphone-based app as a way for parents to report ADHD symptoms and behavior in children with neurodevelopmental conditions. David Hessl, professor in the Department of Psychiatry and Behavioral Sciences runs T-PAL.
-
Hispano-American Brain Bank for Neurodevelopmental Conditions (CENE)
Learn moreEn EspañolCENE collects brains from patients diagnosed with autism, fragile X Syndrome, Down syndrome and other neurodevelopmental disabilities. Our program also performs research, promotes education, and enhances public awareness about the importance of human tissue availability for scientific research on brain function.